A 2.05 M Aqueous Solution
 Multiple Choice Questions
Multiple Choice Questions
21.
18 grand of glucose (C6H12O6) is added to 178.two 1000 of water. The vapour pressure of water for this aqueous solution at 100o C is
-
759.00 Torr
-
7.threescore Torr
-
76.00 Torr
-
76.00 Torr
C.
76.00 Torr

22.
A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol–1) in the same solvent. If the densities of both the solutions are assumed to be equal to i.0 gcm–3, molar mass of the substance will be –
-
xc.0g mol–one
-
115.0g mol–1
-
105.0g mol–1
-
105.0g mol–one
D.
105.0g mol–1
Solutions with the same osmotic pressure are isotonic
Let the molar mass of the substance be M
πone =ConeRT=C2RT =πtwo
So, C1 = C2
As density of the solutions are same

23.
The density (in yard mL–1) of a 3.60 G sulphuric acrid solution that is 29% HiiSo4 (Tooth mass = 98 m mol-) past mass volition be
-
1.64
-
1.88
-
1.22
-
1.22
C.
1.22
Let the density of solution be 'd'
Molarity of solution given = 3.6
i.e. one litre of solution contains iii.half-dozen moles of HiiAnd thenfour
or 1 litre of solution contains 3.6 × 98 gms of HtwoSO4
Since, the solution is 29% by mass.
100 gm solution contains 29 gm HiiSOfour
100/d ml l solution contains 29 gm of H2SO4.
1000 ml solution contains 3.6 × 98 gm of HiiSo4

d = 1.22
24.
The solubility product of a common salt having general formula MXtwo, in water is 4 10 10-12. The concentration of M2+ ions in the aqueous solution of the salt is
-
ii.0 x x-6 M
-
one.0 10 10-4 M
-
i.vi x 10-4 M
-
i.six ten 10-iv Thou
B.
1.0 x 10-iv Yard

25.
At eightyo C, the vapour pressure of pure liquid 'A' is 520 mm Hg and that of pure liquid 'B' is 1000 mm Hg. If a mixture solution of 'A' and 'B' boils at fourscoreo C and 1 atm pressure, the amount of 'A' in the mixture is (i atm = 760 mm Hg)
-
52 mol percent
-
34 mol percent
-
48 mol pct
-
48 mol percent
D.
48 mol percent
PT = PoTenA + Po10B
760 = 520XA+ PoB(1-10A)
⇒ = 0.5
Thus, mole% of A = l%
26.
The volume of a colloidal particle, 5C as compared to the volume of a solute particle in a true solution FiveS, could be
C.
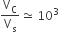
27.
Density of a ii.05 One thousand solution of acetic acid in h2o is 1.02 chiliad/mL. The molality of the solution is
-
1.14 mol kg–i
-
3.28 mol kg–1
-
2.28 mol kg–1
-
2.28 mol kg–1
28.
Equal masses of methane and oxygen are mixed in an empty container at 25ºC. The fraction of the total pressure level exerted by oxygen is
-
2/3
-
(1/three) 10 (273 / 298)
-
one/iii
-
1/three
C.
one/three
Permit the mass of methyl hydride and oxygen is w
mole fraction of oxygen

Let the total force per unit area be P The pressure exerted by oxygen (fractional force per unit area) = 10O2 × Ptotal
P =1/iii
29.
The vapour pressure of h2o at twentyoC is 17.5 mm Hg. If 18 g of glucose (C6H12Ohalf-dozen) is added to 178.two g of water at xxoC, the vapour pressure level of the resulting solution will be
-
17.675 mm Hg
-
15.750 mm Hg
-
16.500 mm Hg
-
16.500 mm Hg
D.
16.500 mm Hg

30.
A mixture of ethyl alcohol and propyl booze has a vapour force per unit area of 290 mm at 300 K. The vapour pressure level of propyl booze is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be
-
350
-
300
-
360
-
360
A.
350
Let the vapour pressure of pure ethyl alcohol be P,
According to Raoult'due south police force 290 = 200 × 0.4 + P × 0.vi
P = 290-lxxx/0.half dozen = 350 mm Hg
- ii
- 3
- iv
A 2.05 M Aqueous Solution,
Source: https://www.zigya.com/competition/jee/study/Chemistry/CH/Solutions/CHENJE12157298/20
Posted by: lilleyhormser.blogspot.com

 Switch
Switch
0 Response to "A 2.05 M Aqueous Solution"
Post a Comment